
 \ Using a mercury manometer, you would need a mercury column at least 152 mm high. What is the total atmospheric pressure of planet Zook? 11.3 mmHg 85.5 mmHg 23.0 mmHg 39.8 mmHg - the answers to Answer: 1 ������������������ question The planet Zook has an atmosphere that contains 3.0 mmHg of neon, 12.0 mmHg of xenon, and 8.0 mmHg of Krypton. According to chemistry, the STP defines a condition at which the temperature of a chemical element (it can be a gaseous chemical element) is equivalent to zero degree Celsius (that is equal to. The unit is named after Evangelista Torricelli, Italian physicist and mathematician, for his discovery of the principle of the barometer in 1643. It is the atmospheric pressure that supports a column of mercury 1 millimetre high. The torr (symbol: Torr) or millimetre of mercury (mmHg) is a non-SI unit of pressure. Task: Convert 975 mmHg to atmospheres (show work) Formula: mmHg ÷ 760 = atm Calculations: 975 mmHg ÷ 760 = 1.28289474 atm Result: 975 mmHg is equal to 1.28289474 atm Conversion Table For quick reference purposes, below is a conversion table that you can use to convert from mmHg to atm. ute and the blood pressure was 105/60 mm Hg. It is measured by two numbers called systolic blood pressure and diastolic blood pressure and is measured in millimeters of mercury (mm Hg). Does the diameter of a tube that contains the mercury affect how high the mercury will rise MMHG Chemistry Abbreviation - All Acronym This constant is specific to the particular gas or mixture (hence its name), while the universal gas constant is the same for an ideal gas Pressure can be measured in m m H g which is equal to the pressure exerted by a column of mercury of a height expressed in m m, at 0 ∘ C under Earth's normal gravity. It is the universal gas constant divided by the molar mass (M) of a pure gas or mixture. Note that rounding errors may occur, so always check the results Video: What is mmHg chemistry? - AskingLot What is the partial pressure of Chemical B water column or mm Hg The SI derived unit for pressure is the pascal. The partial pressure of Chemical A is 24.5 mmHg and its mole fraction is.145. This solution is comprised of Chemical A and Chemical B. Why is this the case and why have people not moved to adopt SI units in this situation A solution's partial pressure is 34.93 mmHg. When medical personnel take a blood pressure reading, such as 115/70 mm HG, it is verbalized as 115 over 70 millimeters of mercury In most areas of chemistry, standard units are adopted - we never would think to use ounces (even American chemists resist) BUT even in modern journals or textbooks, we commonly still see the unit mmHg used. This unit is used in the measurement of blood pressure. According to the American Heart Association, MMHG, commonly written as mm HG, stands for millimeters of mercury. Mercury barometer A millimetre of mercury is a manometric unit of pressure, formerly defined as the extra pressure generated by a column of mercury one millimetre high, and currently defined as exactly 133.322 387 415 pascals. equals 760.0 mm Hg, so there will be a multiplication or division based on the direction of the change Converting between atmospheres and millimeters of mercury.
\ Using a mercury manometer, you would need a mercury column at least 152 mm high. What is the total atmospheric pressure of planet Zook? 11.3 mmHg 85.5 mmHg 23.0 mmHg 39.8 mmHg - the answers to Answer: 1 ������������������ question The planet Zook has an atmosphere that contains 3.0 mmHg of neon, 12.0 mmHg of xenon, and 8.0 mmHg of Krypton. According to chemistry, the STP defines a condition at which the temperature of a chemical element (it can be a gaseous chemical element) is equivalent to zero degree Celsius (that is equal to. The unit is named after Evangelista Torricelli, Italian physicist and mathematician, for his discovery of the principle of the barometer in 1643. It is the atmospheric pressure that supports a column of mercury 1 millimetre high. The torr (symbol: Torr) or millimetre of mercury (mmHg) is a non-SI unit of pressure. Task: Convert 975 mmHg to atmospheres (show work) Formula: mmHg ÷ 760 = atm Calculations: 975 mmHg ÷ 760 = 1.28289474 atm Result: 975 mmHg is equal to 1.28289474 atm Conversion Table For quick reference purposes, below is a conversion table that you can use to convert from mmHg to atm. ute and the blood pressure was 105/60 mm Hg. It is measured by two numbers called systolic blood pressure and diastolic blood pressure and is measured in millimeters of mercury (mm Hg). Does the diameter of a tube that contains the mercury affect how high the mercury will rise MMHG Chemistry Abbreviation - All Acronym This constant is specific to the particular gas or mixture (hence its name), while the universal gas constant is the same for an ideal gas Pressure can be measured in m m H g which is equal to the pressure exerted by a column of mercury of a height expressed in m m, at 0 ∘ C under Earth's normal gravity. It is the universal gas constant divided by the molar mass (M) of a pure gas or mixture. Note that rounding errors may occur, so always check the results Video: What is mmHg chemistry? - AskingLot What is the partial pressure of Chemical B water column or mm Hg The SI derived unit for pressure is the pascal. The partial pressure of Chemical A is 24.5 mmHg and its mole fraction is.145. This solution is comprised of Chemical A and Chemical B. Why is this the case and why have people not moved to adopt SI units in this situation A solution's partial pressure is 34.93 mmHg. When medical personnel take a blood pressure reading, such as 115/70 mm HG, it is verbalized as 115 over 70 millimeters of mercury In most areas of chemistry, standard units are adopted - we never would think to use ounces (even American chemists resist) BUT even in modern journals or textbooks, we commonly still see the unit mmHg used. This unit is used in the measurement of blood pressure. According to the American Heart Association, MMHG, commonly written as mm HG, stands for millimeters of mercury. Mercury barometer A millimetre of mercury is a manometric unit of pressure, formerly defined as the extra pressure generated by a column of mercury one millimetre high, and currently defined as exactly 133.322 387 415 pascals. equals 760.0 mm Hg, so there will be a multiplication or division based on the direction of the change Converting between atmospheres and millimeters of mercury. 
Furthermore, the millimetre of mercury is still routinely used in medicine, aviation, meteorology, and many other scientific fields millimeters of mercury (symbol = mmHg) Pascals (symbol = Pa) or, more commonly, kiloPascals (symbol = kPa) I. In this the mm represents millimetre and Hg is the chemical name of mercury or the symbol for mercury.

What does MMHG stand for in Chemistry? Get the top MMHG abbreviation related to Chemistry Since gradient compression hosiery exert a gradually decreasing amount of pressure up the leg, this unit of measure helps express the amount of pressure the wearer will feel Chemistry MMHG abbreviation meaning defined here.
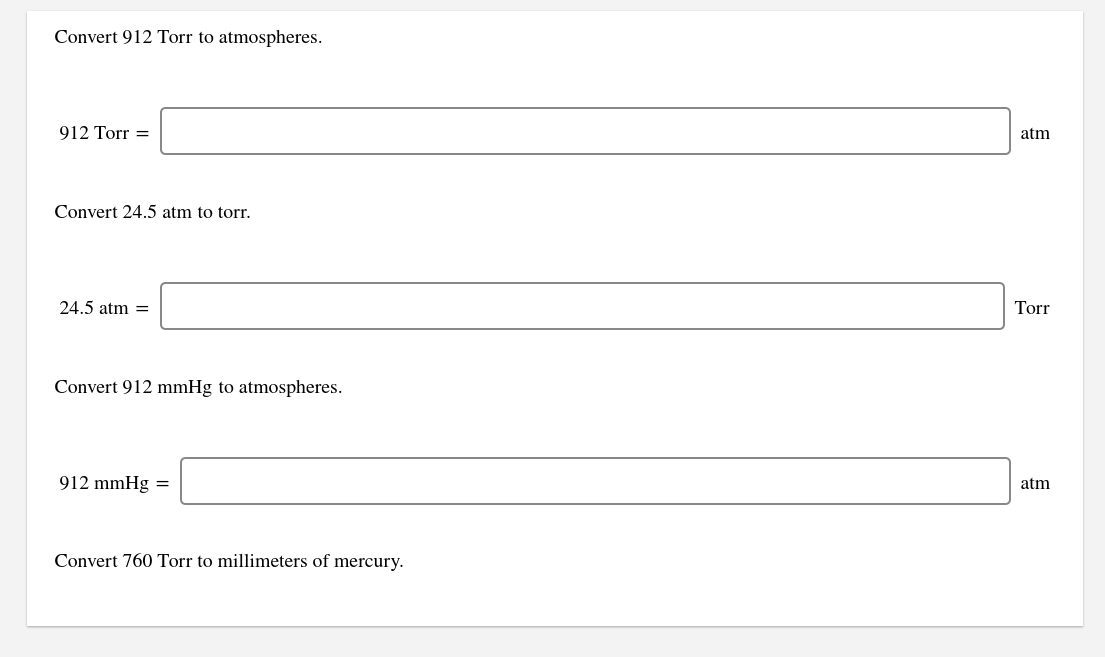
This measurement is used to measure pressure.








 0 kommentar(er)
0 kommentar(er)
